-
Discovery Science
Cellular “Cleaners” More Active in Quiet Brains
Like a robot vacuum for your brain, immune cells called microglia move around and hoover up the neuro-equivalent of crumbs and dirt. But for the first time Mayo researchers have shown in mice that these immune cells of the central nervous system are more active when the brain is quiet than when it is awake.
Their work, published alongside an article with complementary findings in Nature Neuroscience, expands understanding of how microglia sense the environment around them. This in turn may help better understand diseases of the central nervous system, the benefit of sleep, and effects of some medications. This landmark study is highlighted by Nature Neuroscience as well as Nature Reviews Neuroscience.

Night Cleaners
Microglia, in general, find, breakdown, and clean up messes — be they from a bacteria, one of our own dead cells or bits and pieces left over from another process in the body. In the brain they get the lay of the land using long, spindle-like “processes” that constantly move around, contacting neurons and sensing the molecular world around them. Here, too, they specialize says Mayo neuroscientist Long-Jun Wu, Ph.D., senior author of the Nature Neuroscience paper.
“Microglia have developed unique specializations which allow them to sense and respond to the language of the neurological world, not just the immunological world,” he says.

His team reports that in addition to typical immune signals, such as chemokines and cytokines, microglia also respond to a neurotransmitter, such as norepinephrine. Neurotransmitters are released across the space between neurons in the brain and cause a reaction we interpret as a muscle movement, mood or memory. Norepinephrine is known to regulate sleep/wake cycles, arousal, and other phenomenon. When an animal is awake, noroepinephrine levels are high compared to when an animal is anesthetized or asleep.
New Approach, New Knowledge
To date, Dr. Wu explains, just about every study of microglial behavior has been performed in a culture dish, a tissue section, or in the living animal under general anesthesia (read more about this history in Nature Neuroscience’s News and Views and Nature Reviews Neuroscience’s Research Highlight). So Dr. Wu’s team, and another group who published at the same time, decided to investigate how microglia function when an animal is awake.
The researchers showed that as norepinephrine levels drop, microglia wake up.

“I was truly surprised to see microglia becoming most active when neurons shut down, almost like they were some form of nocturnal cell type,” says Yong Liu, Ph.D., the first author of the this work. “After testing a number of molecules that could potentially regulate this transition, we were intrigued to find norepinephrine signaling was at the heart of this change.”
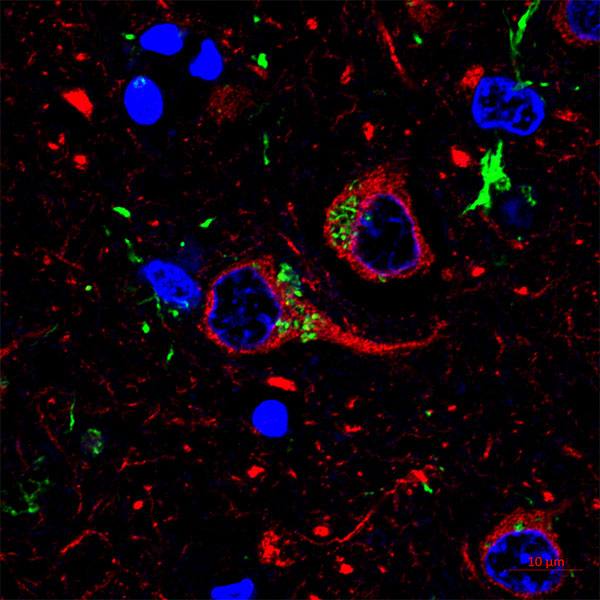
The underlying mechanism driving microglial process activity was expression of a receptor for norepinephrine. Using a number of methods, the team locally or globally reduced the activity of neurons and in response, microglia processes began to move at greater velocities, expand outward, and occupy a larger volume of the brain parenchyma.
“They extend their processes, survey the environment around them, and make more prolonged contact with neurons,” says Dr. Wu. “What is exciting but not yet known is if this increase in activity and its interaction with neurons allows microglia to make subtle changes to how neurons fire or connect to one another.”

Future Research: Sleep, Medication, Disease
Another area of potential interest for Dr. Wu and his team is sleep.
“We know memory consolidation can occur during certain sleep stages, but we do not know whether microglia are involved in that process,” he says. The team is hoping to understand whether increased microglial movement and contact with neurons occurs during sleep stages associated with memory consolidation. They are also interested in how this finding relates to diseases and other treatments for disease.
“For example, patients take beta blockers every day to reduce high blood pressure or to treat/prevent a range of other symptoms,” says Dr. Wu. “One such drug, propranolol, was used in our study to prevent microglia from altering their activity. An interesting question is to determine how long-term administration of such drugs alters the brain environment, as seen through the lens of microglial interactions.”
Also, abnormal norepinephrine levels can exist in many different disease contexts, such as epilepsy, pain, autoimmune disorders and forms of neurodegeneration. The team plans to look at if those levels contribute to disease through a microglial mechanism.